Help – Start an Industry-Sponsored Clinical Trial Proposal
Definitions
Industry Sponsored Clinical Trial Proposal Template: The proposal template used for proposals using program type “Industry Sponsored Clinical Trial” or “Non-Interventional, Patient Registry Study, Point of Care Device Validation Study.”
Program Type: A defining categorization of the nature of the project; historically called Chess Code
Industry Sponsored Clinical Trial: A research study, funded by a for-profit company that is engaged in the development, manufacture, testing, and/or distribution of pharmaceuticals or medical devices intended for human use, in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
- Prospectively assigned refers to a pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo or other control) of the clinical trial.
- An intervention is a manipulation of the subject or subject’s environment for the purpose of modifying one or more health-related processes and/or endpoints. Examples include: drugs/small molecules/compounds; biologics; devices; procedures (e.g., surgical techniques); delivery systems (e.g., telemedicine, face-to-face interviews); strategies to change health-related behavior (e.g., diet, cognitive therapy, exercise, development of new habits); treatment strategies, prevention strategies, and diagnostic strategies.
- A health-related biomedical or behavioral outcome is the pre-specified effect of an intervention on the study subjects. Examples include positive or negative changes to physiological or biological parameters (e.g., improvement of lung capacity, gene expression); psychological or neurodevelopmental parameters (e.g., mood management intervention for smokers; reading comprehension and/or information retention); disease processes; health-related behavior; and, well-being or quality of life.
Non-Interventional/Patient Registry/Point of Care Device Study: a study conducted to assess safety, tolerability, and effectiveness of marketed medicines in clinical practice, i.e. in a naturalistic setting where the choice of therapy is consistent with approved marketing authorization and in line with the current standard of practice. Patient selection and the diagnostic or monitoring procedures are those applied per the usual treatment paradigm of the treating physician and not per protocol. A patient registry study uses observational (not interventional) study methods to collect uniform data (clinical and other) in an organized system and to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves a predetermined scientific, clinical, or policy purpose(s). A point of care device validation study tests the clinical analytical performance of a new device against the relevant standard of care diagnostic test.
Clinical Trial Anticipated Revenue: revenue anticipated across the life of the clinical trial
Procedure
Navigate to the Proposal Development module and
- click Proposal under the Create New section
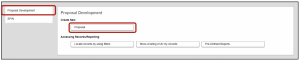
- In the New Proposal Questionnaire:
- Select Create New Proposal
- Click Continue
- Select Setup Proposal Manually
- Click Continue
- Select New/Original
- Continue
- Select Create New Proposal
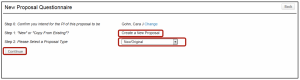
- Type Sponsor Name
- Click Continue
- Type Proposal Title
- Continue
- Enter Project Start and End Dates
- Continue
- Change the number of periods to = 1
- Continue
- Click Create Proposal
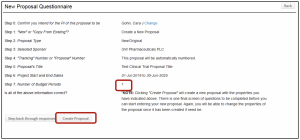
- On the Setup Questions page:
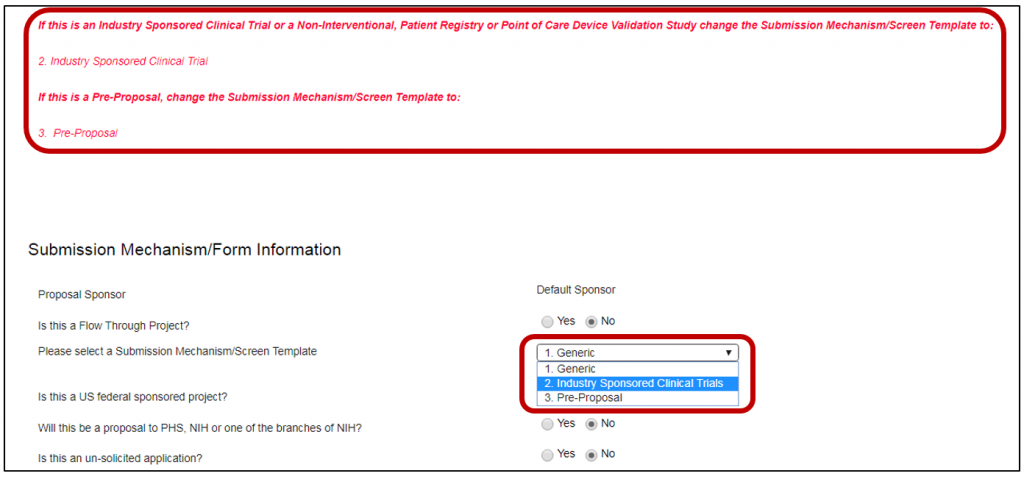
- Instructions are included in red text about the different proposal templates available
- Scroll to Please select a Submission Mechanism/Screen Template
- Select 2. Industry Sponsored Clinical Trial
-
- Click OK in the confirmation window (pop up)
- After the template refreshes, enter these parameters:
- Is this a Flow Through Project – A “Yes”
- Designate originating sponsor in pop-up
- Deadline Information – defaults to having no submission deadline
- Associated Departments – See Associated Departments Training Document for guidance
- Associated Centers/Programs – See Associated Departments Training Document for guidance
- Select Program Type – Clinical Trial – Industry Sponsored or Non-Intervnetional, Patient Registry Study, Point of Care Device Validation Study
- NOTE: If neither of these program types is appropriate, change the proposal template to 1. Generic and proceed with proposal development
- The majority of the research will be conducted – Defaults to On Campus
- Is this a Flow Through Project – A “Yes”
- Click Save
- Complete the Setup Questions:
- Click the Completed check box in the upper right-hand corner
- Click Ok
Navigate and complete the following tabs (refer to those help pages if necessary):
- Pre-Review Info
- Pre-Review Routing/Upload
- Additional Proposal Information
- Personnel
- F&A Dist/Credit Allocation
On the Budget tab:
- See help page on Completing the Personnel, Budget & Allocations Tab training document
- The notes below provide additional summary:
- Add personnel using the search box under the Personnel section
- Choose Key or Non-Key
- Choose the Role
- Click Add Person
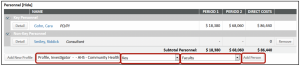
- Add effort % or person months
- Add effort without populating salary dollars by navigating to the Appointments tab and deleting the base salary > Save
- Then, enter the effort on the Detail tab
- Save
- Committed effort will be captured without the salary calculations.
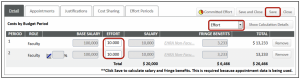
- On the Non-Personnel section
- Choose category Total Projected Cost (CT Use Only)
- Click Add Item
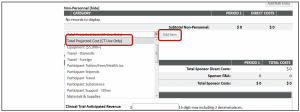
- Type in the appropriate amount minus F&A in the pop-up window
- Click Save and Close
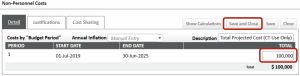
- On the F&A tab of the budget screen:
- The F&A will pre-populate based on the Program Type chosen in the Setup Questions
- Click Apply
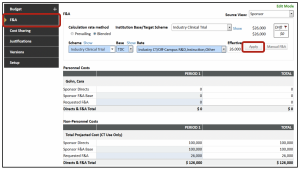
- Complete any other tabs in the budget screen
- See “Completing the Personnel, Budget & Allocations Tab” training document
- On the Summary Budget tab
- Click Complete Budget
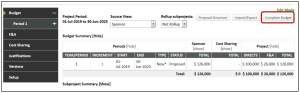
- Click Done in the upper left-hand corner
On the Finalize screen
- Click Build in the Build PDF/Form Pages section
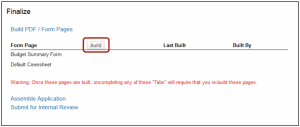
- Click Build in the Assemble Application section

- Find a PDF of the proposal components in the Form/Document sections labeled Assembled Doc
- View the assembled document using the glasses icon.
![]()
- Click Submit
- The proposal will enter approval route